The Goals of Bench Research
- Is ascension of vaginal microbes necessary and/or sufficient for sPTB?
- What are the molecular and immunological mechanisms by which non-optimal microbes induce premature cervical remodeling (biomechanically) through disruption of the cervical epithelial barrier?
- How does microbiome-epithelial crosstalk modulate cervical remodeling and/or allow for ascension of microbes?
- What are novel ways by which microbes may communicate with reproductive tissues?
Intrauterine Inflammation and fetal and childhood outcomes:
Inflammation at the maternal-fetal interface during pregnancy represents a significant risk factor for adverse neurobehavioral outcomes, including cognitive delay, schizophrenia, autism and mental retardation. A variety of antenatal infections and inflammatory stimuli have been demonstrated to induce common adverse outcomes specifically fetal and neonatal brain injury. Our central hypothesis is that a variety of inflammatory exposures at the level of the uterus induce shifts in the immune cell composition and immune function in the decidua which in turn alter the immune cell composition and function in the placenta. These immunological events at the maternal-fetal interface are critical to inducing fetal brain injury. This hypothesis is based on both animal and human data that have demonstrated that exposure to prenatal inflammation results in fetal brain injury that persists postnatally and results in a spectrum of diverse neurobehavioral phenotypes.
Our published work has fully characterized a mouse model of local intrauterine inflammation that closely recapitulates the human condition of prenatal inflammation and spontaneous preterm birth (sPTB). With these models, we have demonstrated that mid-gestation exposure to prenatal inflammation results in brain injury antenatally and postnatal changes in neurogenesis which manifest in behavioral deficits. A critical gap in knowledge is what are the precise mechanisms of this brain injury? This knowledge gap is quite evident in our lack of any effective therapeutic strategies to decrease fetal or neonatal brain injury in the setting of prenatal inflammation. Our current research focuses on elucidating how shifts in immune cell composition and function at the maternal-fetal interface are critical in the pathogenesis of fetal brain injury in the setting of prenatal inflammation.
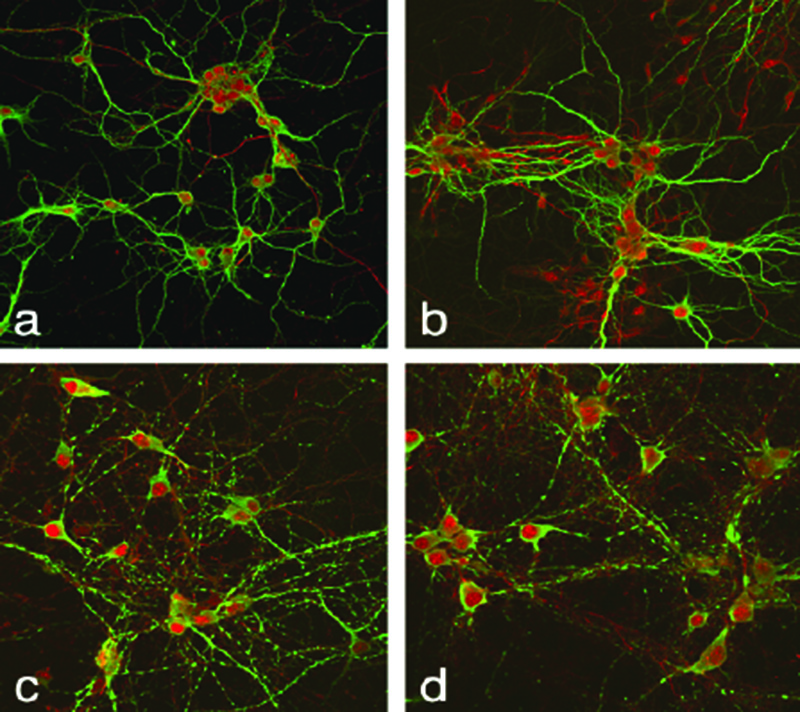
The role of immune cells at the maternal-fetal interface in normal and abnormal pregnancy:
Mammalian pregnancy requires the maternal immune system to tolerate a semi-allogenic fetus. At the same time, the immune response must protect the mother (and fetus) against infection. Thus, the placenta, as an immunological organ, must serve both roles. We hypothesize that it is in the capacity of having to protect and defend, that the immunological signals from the placenta in the setting of intrauterine inflammation may protect the mother but harm the fetus. Some studies in human pregnancy describe the presence of different immune cells in the placenta and how they differ after exposure to intrauterine inflammation. However, our current knowledge of which immune cells are present at the maternal-fetal interface over time at how intrauterine inflammation alters the composition or function of those cells is severely limited. Similarly, while it is known that fetal cells can traffic to the maternal side, there is very limited data on whether maternal cells can traffic (can have a functional effect) to the fetus.
What are the immune cells at the maternal fetal interface in normal pregnancy?
Much of what is known regarding immune cells at the maternal fetal interface in human pregnancy is from studying decidua/placentas from after delivery and/or using findings in peripheral blood and assuming it represents local immune cell composition. Studies suggest that key players, in terms of immune cells for normal pregnancy, include natural killer (NK) cells, CD8+ T cells, dendritic cells (DCs), gamma/delta (g/d) T cells, and regulatory T cells (Tregs). Understanding the inability to test the maternal-fetal interface during a human pregnancy, animal work has provided more insight into the immune cell composition in different tissues across time. We recently reported on the spatiotemporal shifts of immune cell composition in the decidua and placenta over the course of a murine gestation.(Lewis et al AJRI 2020). The primary finding was a significant decrease in Tregs in the placenta just prior to term parturition. This finding is consistent with a reported role of Tregs in pregnancy maintenance. While these studies begin to characterize immune cell composition and functions at the maternal fetal interface in normal pregnancy, they do not address what occurs in the setting of an inflammatory challenge.
FIGURE TBD
Paradigms for spontaneous preterm birth (sPTB):
A central paradigm for sPTB is that CV microbes ascend above the cervix to cause chorioamnionitis and intrauterine inflammation. While chorioamnionitis is certainly one cause of sPTB, not all episodes of sPTB can be attributed to chorioamnionitis. Multiple clinical trials have been performed to target infection during pregnancy, but have all failed to decrease sPTB rates. The failure of these clinical trials have prompted some to question the accepted paradigm of sPTB. As such, an alternative paradigm for how CV bacteria cause sPTB has emerged which postulates that a key initiator of sPTB is premature cervical remodeling. The premise of this paradigm is that the interaction of CV microbiota with the epithelial barriers drives premature cervical remodeling. There are now several lines of evidence, several generated by the Elovitz Lab, that support this paradigm. A cornerstone to this paradigm are human studies demonstrating an association between the CV microbial communities and PTB. Prior studies addressing this association have been limited by small sample sizes, heterogeneity in phenotyping and different methodological approaches. To address this question with increased rigor, we completed a 2000 woman cohort that demonstrates strong associations between CV microbiota and sPTB. Further supporting this alternate paradigm of sPTB is in vivo and in vitro work that demonstrates that disruption of the cervical epithelial barrier is involved in cervical remodeling.28,29,31,36,44,45 Additionally, in vivo work from our laboratory, demonstrates that specific high-risk bacteria, such as G. vaginalis and M. mulieris, can induce sPTB in mice whereas Lactobacillus does not.28,45 Collectively, this body of research begins to ascribe mechanisms for the observed association between microbial communities and sPTB in human cohort studies. What remains to be studied is how microbial communities in the CV space induce premature cervical remodeling and contribute to sPTB in human pregnancy.
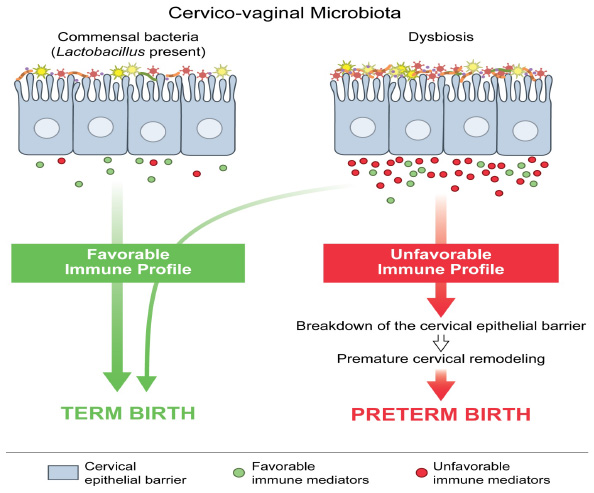
The CV space as a unique biological niche: This alternative paradigm of sPTB implores a better understanding of the CV space. The vagina is an elastic muscular canal that is covered in non-keratinized stratified squamous epithelium. The epithelial covering of the cervix is continuous with the vagina but the epithelial type changes. Structurally, the cervix is compromised of the ectocervix which is lined by squamous cells and the endocervix which is lined by columnar glandular cells (Figure 1). The different epithelial cells have their own immune cell repertoires.47,48. The concept that the CV space is merely a conduit for bacteria to have entry into the uterine cavity is oversimplified. Similar to what occurs in many other biological niches where it is known that the microbiota influence health and disease through changes in metabolites, microbial and host gene expression and varying immune responses, the CV space must have its own unique ecosystem that drives health and disease. Published data from our group demonstrate the molecular and metabolic changes that are present in the CV space and with sPTB. Current fundamental and translational research in the Elovitz Lab are leveraging these discoveries to better understand the CV ecosystem in sPTB.
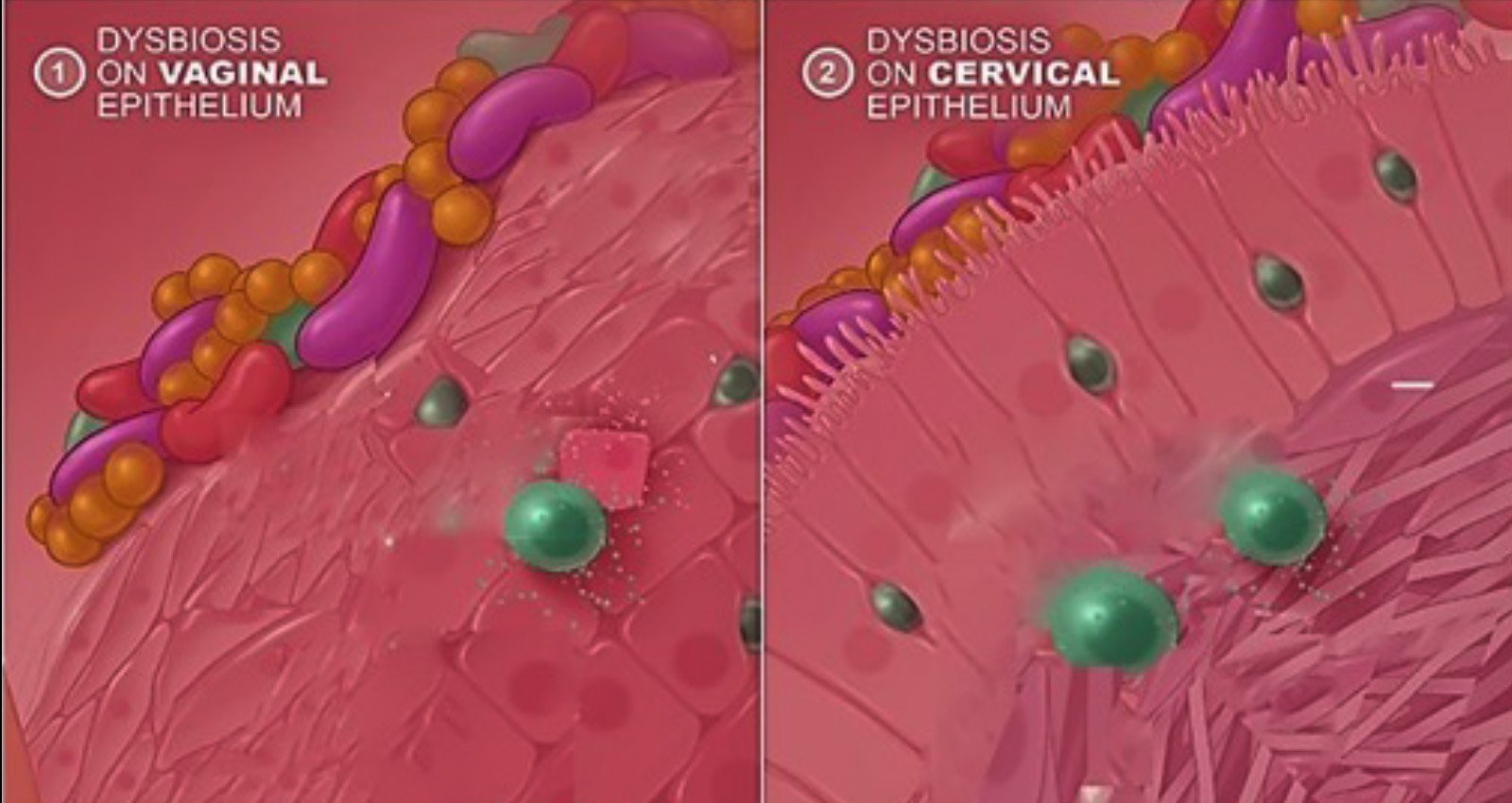
Graphical representation of the microbiota covering the vaginal epithelium (1) and the cervical epithelium (2). Metabolities and immune Mediators are noted throughout the space.
- Elovitz MA, Gajer P, Riis V, Brown AG, Humphrys MS, Holm JB, Ravel J. Cervicovaginal microbiota and local immune response modulate the risk of spontaneous preterm delivery. Nat Commun. 2019 Mar 21;10(1):1305. doi: 10.1038/s41467-019-09285-9. PMID: 30899005; PMCID: PMC6428888.
- Gerson KD, McCarthy C, Elovitz MA, Ravel J, Sammel MD, Burris HH. Cervicovaginal microbial communities deficient in Lactobacillus species are associated with second trimester short cervix. Am J Obstet Gynecol. 2020 May;222(5):491.e1-491.e8. doi: 10.1016/j.ajog.2019.11.1283. Epub 2019 Dec 6. PMID: 31816307; PMCID: PMC7196011.
- Anton L, Sierra LJ, DeVine A, Barila G, Heiser L, Brown AG, Elovitz MA. Common Cervicovaginal Microbial Supernatants Alter Cervical Epithelial Function: Mechanisms by Which Lactobacillus crispatusContributes to Cervical Health. Front Microbiol. 2018 Oct 8;9:2181. doi: 10.3389/fmicb.2018.02181. PMID: 30349508; PMCID: PMC6186799.
- Dude CM, Saylany A, Brown A, Elovitz M, Anton L. Microbial supernatants from Mobiluncus mulieris, a bacteria strongly associated with spontaneous preterm birth, disrupts the cervical epithelial barrier through inflammatory and miRNA mediated mechanisms. Anaerobe. 2020 Feb;61:102127. doi: 10.1016/j.anaerobe.2019.102127. Epub 2019 Nov 21. PMID: 31760081.
Ghartey J, Anglim L, Romero J, Brown A, Elovitz MA. Women with Symptomatic Preterm Birth Have a Distinct Cervicovaginal Metabolome. Am J Perinatol. 2017 Sep;34(11):1078-1083. doi: 10.1055/s-0037-1603817. Epub 2017 Jun 12. PMID: 28605823.
Racial disparity in sPTB: There are enormous racial disparities in sPTB prevalence in the United States. Despite decades of research and public health interventions, Black women have a 50% higher sPTB risk, leading to a corresponding increased risk of black infant mortality. Epidemiological data supports the concept that racial disparities in health outcomes are at least partially linked to psychosocial stress related to being a member of a discriminated racial minority group which includes depressed mood, anxiety, perceived racism, and adverse childhood experiences and that these factors contribute to health disparities in Black women. Due to longstanding residential racial segregation in the U.S., health disparities, including disproportionately high rates of PTB, among Black women may be, in part, attributable to neighborhood factors, and perceived racism. Neighborhood deprivation is more common in black urban areas and has been shown to be associated with PTB. Both perceptions of racial discrimination and maternal stress have also been linked to sPTB. Adverse childhood experiences (ACE) are prevalent in pregnant women and have been associated with PTB. It is biologically plausible that psychosocial exposures and behaviors influence the cervicovaginal ecosystem and sPTB in Black women. Racial disparities in sPTB represent one of the largest disparities in human health in our country and the gaps are only widening. Addressing this evolving health care crisis, research in the Elovitz Lab is centered on sPTB among Black women with the goal of discovering modifiable factors, both psychosocial and biological, that identify who are at greatest risk for sPTB—thus allowing discovery therapeutic targets to reduce this significant adverse health outcome for a high-risk population.





